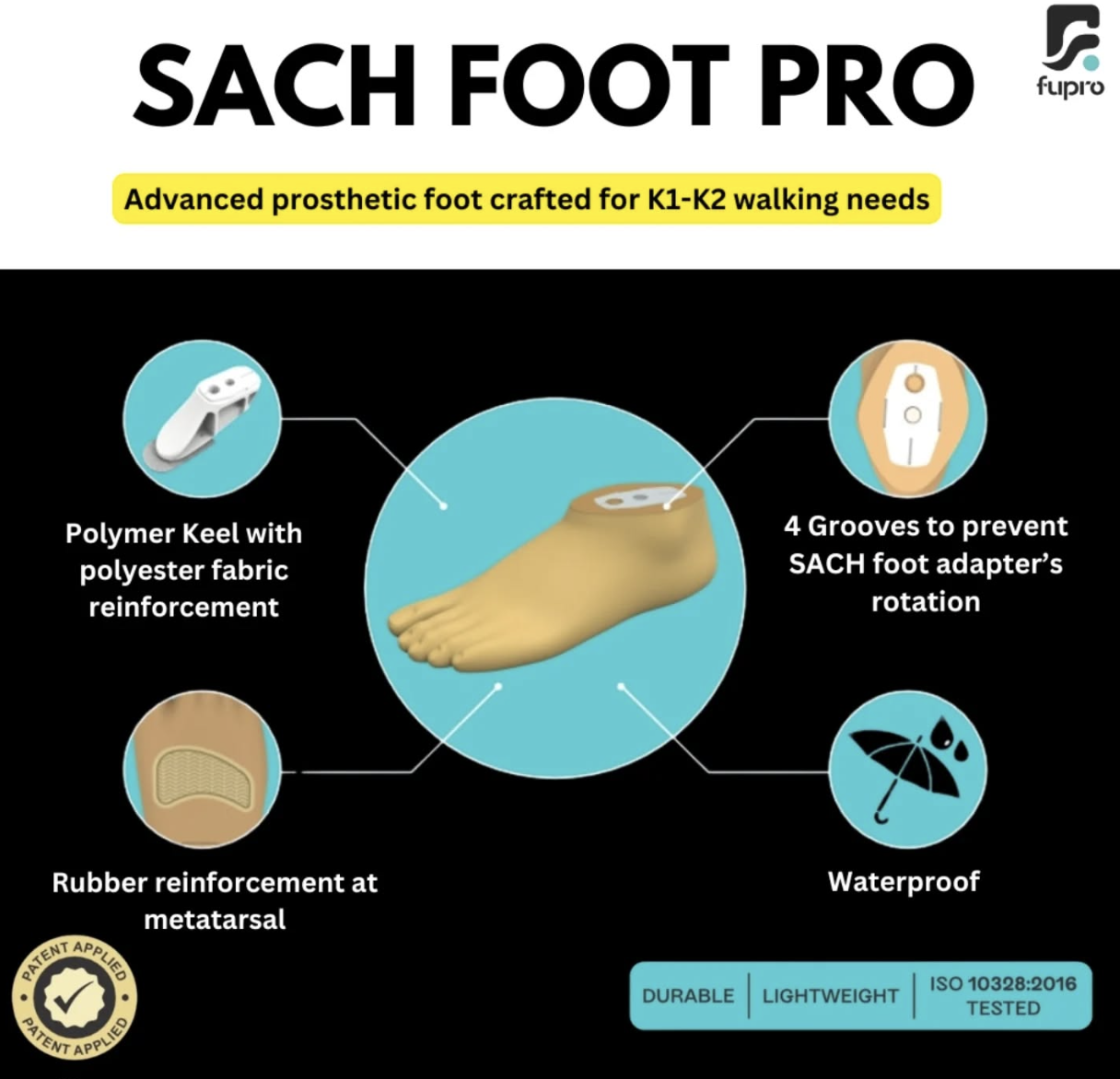
Nine tips for improvements in patient outcomes, function and materials when 3D printing O&P devices.
By Melanie Shelton, HP Inc.

Edser used 3D printing for this DAFO (dynamic ankle foot orthosis) device. [Image courtesy of HP Inc.]
For O&P companies to harness the potential of 3D printing, they need strategies that optimize patient outcomes, improve device functionality and maximize 3D-printed material strength, reliability and durability.
Patient outcome improvements
1. Plan in advance: Effective 3D printing begins with meticulous planning. Gone are the days when designers and engineers could get away with applying traditional manufacturing methods to 3D printing and call it good. Force-fitting old ways into new technology will not work. To be effective, it’s important to adopt a 3D mindset and take a unique approach while planning for each project.
Software such as Leopoly’s LeoEngine can help facilitate the automation of various design and fabrication steps, enhancing the efficiency and scalability of device designs. Developers of the Arize Orthotic Solution, an end-to-end digital orthotic service for podiatrists and orthotists, used design automation to reduce lab review time. Automation can significantly improve manufacturing and delivery times for less complex medical devices such as foot orthoses or ankle-foot orthoses (AFOs).
Computational design, which uses algorithms to optimize and automate processes, can also significantly reduce the time and effort of creating custom devices. Smith Optics uses computational design to efficiently create custom-fit ski goggles from facial scans.
2. Think customization from the start: Customization is what 3D printing is all about when it comes to delivering better patient outcomes in O&P. Rather than initially thinking about ways to mass produce artificial ankles, knees, feet, insoles, or spinal, wrist and hand supports, better patient outcomes are achieved by identifying ways to tailor 3D-printed devices to the needs and anatomy of specific patients or patient groups. If those groups are large enough, designs can be extended to accommodate the needs of those patient segments.
3. Integrate smart features: Incorporating smart features into 3D-printed devices can further enhance patient care. Embedded sensors and connectivity options will eventually enable proactive remote monitoring and data-driven insights to improve patient comfort and track O&P device performance, whether it might be about to fail, how the patient’s anatomy is interacting with it, how much pressure it’s withstanding each day and even how active the patient has been since wearing the device. This built-in feedback loop allows for improvements in each generation of a device to be constructed using real world data.
Functional improvements

All 3D Labs used 3D printing to make this arm cast. [Image courtesy of HP Inc.]
While there is no single set of DfAM principles, they generally involve part consolidation, build packing optimization, design freedom and biomimicry. Look into comprehensive training courses to bring designers and engineers up-to-speed on DfAM.
5. Conduct iterative prototyping: Iterative prototyping and testing is vital to refining the functional aspects of 3D-printed devices. This process allows designers to make incremental improvements based on real-world feedback and performance data. By continuously iterating designs, manufacturers can enhance device performance and address specific patient needs more effectively.
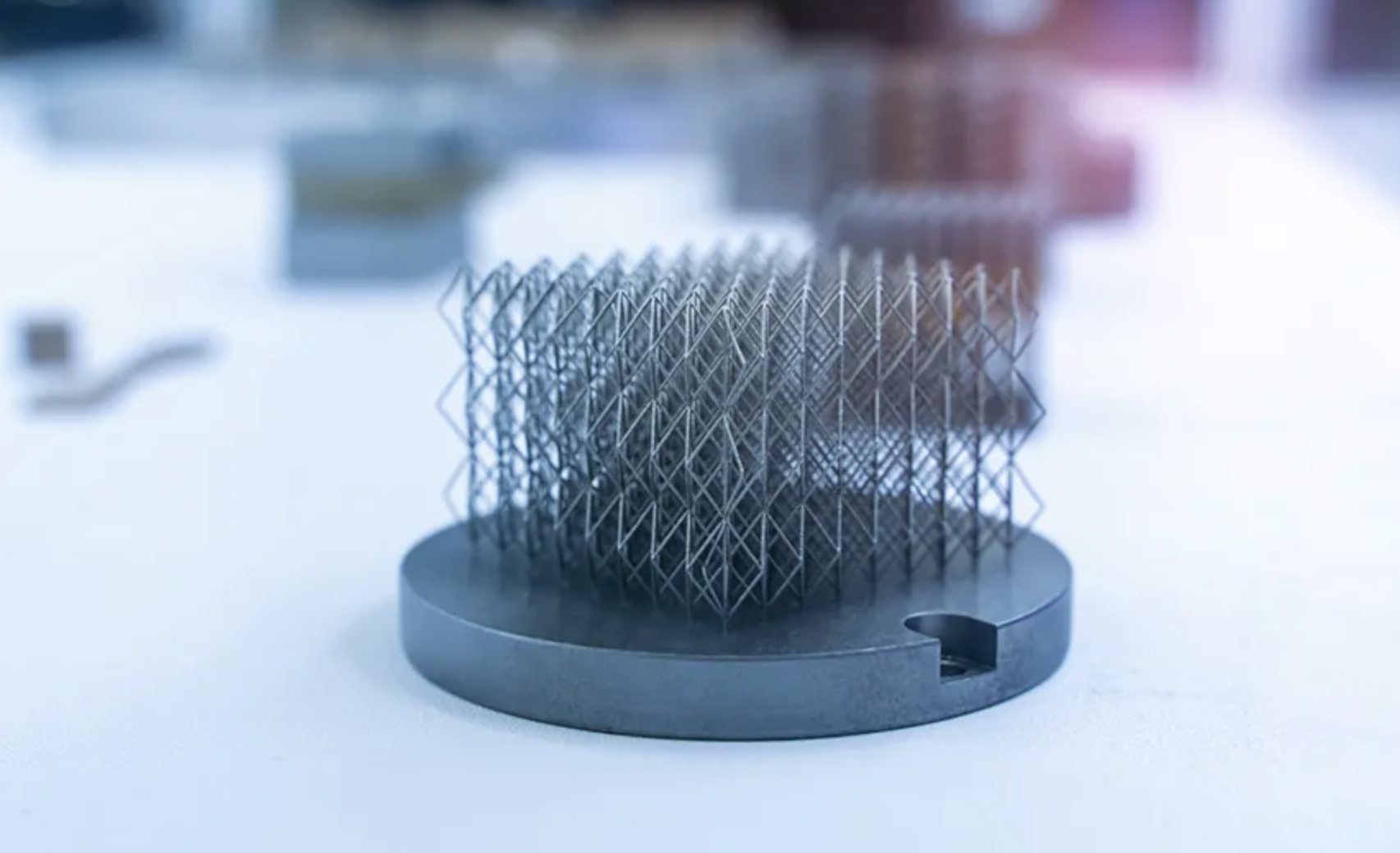
6. Incorporate complex structures: Exploring and incorporating complex structures into device design can address functional requirements in innovative ways, particularly for those that exhibit meta-material properties and can behave in both characteristic and useful ways based on the material’s sub-structure. Indeed, small adjustments to reduce weight, disperse heat, or manage pressure distribution can improve a device while creating revenue opportunities. Click Medical recently secured HCPCS Code L5783 certification from the Centers for Medicare & Medicaid Services for insurance coverage and reimbursement eligibility.
Material improvements
7. Understand material attributes: When imagining 3D-printed devices, a designer needs a working knowledge of material types, their capabilities and limitations and general availability before getting started. It’s a mistake to assume materials used in traditional manufacturing will be just as applicable to additive manufacturing.
Common 3D printing materials fall into three categories: plastics/polymers, metals and ceramics. Each offers varying durability, reliability, elasticity, heat resistance, biocompatibility and sustainability. You’ll need to select materials to support specific projects rather than hastily buying good-enough options that may be more affordable or have fared well on prior manufacturing jobs. HP offers a guide to 3D printing materials.
8. Enhance material properties: Enhanced or specialized materials knowledge should also be part of a device designer’s toolkit. Chemical vapor smoothing, for example, is a post-processing method that uses a combination of heat and solvent vapor to improve the mechanical properties of parts and products. Materials with high abrasion resistance, like ESTANE 3D TPU M95A, can also be useful for jigs and fixtures that undergo thousands of cycles. Multi Jet Fusion (MFJ) technology needs less heating power than selective laser sintering (SLS) systems and can improve material properties while reducing waste. Finally, biocompatible materials like polylactic acid (PLA) or BioMed Amber Resin can ensure patient safety and compliance with FDA regulations.
9. Research novel materials: Novel materials may be essential for addressing specific medical needs. MJF technology often uses PA11 or PA12 polyamide plastics, which are sometimes compared to sheet-source polypropylene found in traditional manufacturing plants but are not all that close. Designers need to understand the differences and choose the right material for the specific job at hand. Collaborating with application engineers and designers can help designers study and understand the interplay of materials, agentsand designs for projects. By leveraging novel materials, design teams can integrate and consolidate functions into a single part, such as adding claps, latches, or other adjustments directly into the printed part.
Final thoughts
3D printing is poised to revolutionize O&P by enabling highly customized, patient-specific devices with intricate geometries and optimized functionalities. Careful planning, meticulous design and strategic material choices will be needed to guide this transformation. By harnessing the power of automation, personalized solutions, smart integrations and cutting-edge DfAM principles, designers can reshape patient outcomes, refine device functionality and raise materials usefulness with unprecedented precision and payoffs for years to come.

Melanie Shelton [Photo courtesy of HP]