The first time Keith Vonderhuevel came home wearing an experimental prosthetic arm, something remarkable happened. He walked through the door, his young granddaughter rushed over to hug him, and—for the first time in years—Vonderhuevel could hug her back without fear of gripping too hard.
“I could tell how hard I was squeezing,” he recalls, smiling at the memory. “It felt like I got my hand back.”
Vonderhuevel lost his right hand in a 2005 work accident. In the ensuing years, he occasionally used conventional prosthetic limbs to help him do basic tasks. But the new device, which he wore as part of a clinical trial at the Cleveland VA Medical Center, enabled Vonderhuevel to “feel” his missing limb.
That’s one of the core features of neuroprosthetic devices. By tapping into the body’s natural neural pathways, they enable a seamless, two-way flow of information between the artificial limb and the wearer’s mind. The most advanced prototypes not only restore the sense of touch, as Vonderhuevel described. They also support intuitive motor control, so wearers can flex an ankle or bend a finger with a simple thought.
Such technology would revolutionize the prosthetics industry and give amputees a level of function that’s only been seen in sci-fi movies. Prosthesis designers have been chasing these goals for most of the 21st century, and they seemed to be getting close when Vonderhuevel began volunteering at the VA back in 2013. In the decade since, our news feeds have buzzed almost nonstop with reports of new, increasingly lifelike neuroprosthetic devices. We read and hear about them all over the place, from scientific journals and academic conferences to Ted Talks, 60 Minutes segments, and popular magazines like Wired and Scientific American (and, yes, Amplitude).
These accounts often give the impression that neuroprosthetic limbs will soon be available in O&P offices everywhere. But they never seem to arrive. They seem to be perpetually trapped in design laboratories and test clinics. What’s the holdup?

The reality is that market-ready neuroprostheses approved by the US Food and Drug Administration (FDA) are still a few years away. But steady technological progress and new frontiers in mind-machine research seem to be accelerating the pace and hastening the day when these revolutionary devices finally reach consumers.
It makes sense that neuroprosthetic limbs will eventually be widely available. Other types of neuroprosthetic technology have been around for decades. Any medical device that restores lost or altered neural function fits the definition. Therefore, pacemakers—which correct dysfunctional nerve activity in the heart—are a type of neuroprosthesis, and they have been in broad usage since the 1960s. Cochlear implants, another type of neuroprosthesis (wired directly into the auditory nerve), hit the consumer marketplace in the mid-1980s. Spinal cord stimulators, which received FDA approval just a few years ago for pain relief, are the latest type of neuroprosthesis to become fairly common in medical practice.
Amputees have worn myoelectric limbs for more than half a century, and it can be argued that these are a type of neuroprosthesis. But myoelectrics don’t operate along intuitive neural routes, so controlling them requires a lot of mental energy. They also don’t simulate a sense of touch.
So myoelectrics are a far cry from the neuroprosthetic limbs currently under development. The newest devices establish a complete neural circuit. They’re equipped with sensors (usually in the fingers for upper-limb devices, and the soles of the feet for lower-limb) that transmit signals back to the nervous system, allowing wearers to “feel” their environment. They also carry high-fidelity signals in the opposite direction, from the mind to the prosthesis, leveraging the body’s natural infrastructure as much as possible so users can control their devices with great precision and low mental effort.
Researchers have devised a range of technical solutions to achieve these effects, including:
- Targeted muscle reinnervation (TMR). First tested in 2002, TMR reroutes nerves that ran to the amputated limb and reintegrates them with remaining muscles. Electrodes on the skin’s surface carry signals back and forth between the nerve endings and the device.
- Composite flat interface nerve electrodes (C-FINEs). These interfaces are implanted below the skin’s surface and wrap around peripheral nerves, creating a direct connection between the nerves and the neuroprosthesis. C-FINEs have been used in lower-limb devices to help users sense whether the surface they’re standing on is hard or soft, level or tilted, and so forth. Vonderhuevel’s prosthetic arm also uses C-FINEs to give him advanced sensation in his fingers.
- Regenerative peripheral nerve interface (RPNI). Similar to TMR, RPNI promotes organized nerve regrowth by implanting severed nerves into muscle tissue. But instead of rerouting severed nerves to a new location, RPNI leaves them in place and grafts muscle tissue over them.
- Agonist–antagonist myoneural interface (AMI). In AMI, paired muscles and nerves in the amputated limb are surgically reconnected, preserving normal neural circuitry. Patients who’ve undergone AMI surgery display a heightened sense of proprioception and advanced control over neuroprosthetic devices.
Vonderhuevel is currently testing a new type of C-FINEs interface for the Cleveland VA. A device embedded in his chest (similar to a pacemaker) wirelessly receives feedback from pressure sensors on the prosthesis and transmits commands back to the device. He uses a smartphone app to fine-tune this system, which gives him precise control over his movements. Using this experimental hand, Vonderhuevel can crack an egg, button his shirt, pour coffee, and maintain eye contact with someone while he’s shaking hands. “I can feel how tight I’m squeezing,” he explains.
Vonderhuevel’s experience illustrates a benefit that nobody saw coming: Sensory-enabled prostheses significantly improve users’ body image, self-esteem, and acceptance of their artificial limbs (also known as embodiment). Instead of viewing their prostheses as inert tools, amputees involved in clinical trials consistently report that neuroprosthetic devices feel more like integral extensions of their bodies. This perception, in turn, boosts their confidence and helps them more fully engage with the world and with other people.
“Almost everyone said they wanted touch because it allowed them to interact with people better,” says biomedical engineer Emily Graczyk, who coauthored a groundbreaking 2018 study that measured the psychosocial advantages of sensory neuroprostheses. “The biggest thing we saw was the emotional impacts and the social impacts, specifically for the sense of touch. For our participants that we’ve had in our studies, they think it’s night and day to have the neuroprosthetic versus a standard prosthetic. They say, ‘I would take the neuroprosthetic every day if I could. I want that.’”
A Team Effort to Get Into O&P Clinics
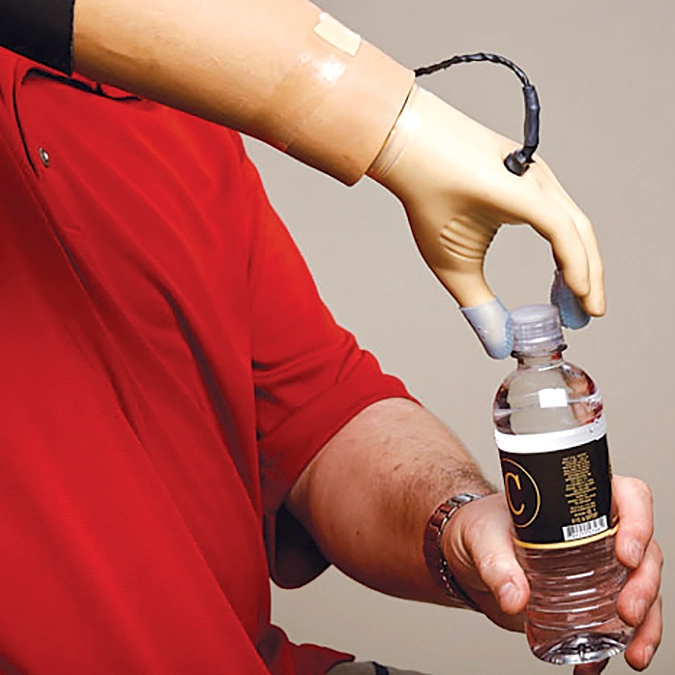
But despite the overwhelming demand for neuroprostheses, only research subjects such as Vonderhuevel have been able to experience the benefits—and almost always within the confines of a lab, rather than in their daily lives. Most amputees are still waiting for their first chance to try one of these brain-powered limbs.
Laurent Frossard, a bionic limb expert from Griffith University in Queensland, Australia, explains that every medical device has to pass through nine technology readiness levels (TRLs) on its way from the lab to the marketplace. This journey typically lasts anywhere from ten to 15 years, and it’s not a linear progression. “Experience shows that the time spend in the early stages of development of neuroprosthetics to reach TRL 3 is extensive,” Frossard says in an email.
The US Department of Defense’s (DOD’s) rubric for biomedical TRLs defines TRL 3 as a proof-of-concept phase that culminates in an initial prototype. Researchers must perform enough analysis to specify the device’s components and materials and conduct enough testing to prove that the device can safely achieve its intended purpose. Completing TRL 3 for a neuroprosthesis requires recording and processing reams of data about the interactions among the brain, nervous system, and muscles, and translating that information into electrical signals to command the prosthesis or interpret incoming sensory feedback.
Once all that front-end development is concluded, there remain six more TRL hurdles that involve standards and compliance reviews, production testing, clinical trials, quality inspection, technical documentation, and on and on. Beyond that, there’s the task of convincing insurers and health systems that the return on investment is worthwhile—all for a product class that’s perceived as a small niche within the medical device industry.
“All the technological hurdles can and will be overcome over time,” Frossard says. “However, the pace of progress largely depends on the amount of funding readily available.”

Research funding often comes with many strings attached, Graczyk adds. The DOD has allocated millions of dollars to support neuroprosthetic research labs such as hers, which is based at Case Western Reserve University and collaborates regularly with the Cleveland VA. Her well-regarded team has achieved numerous breakthroughs, including an advanced C-FINE apparatus that ranks as the longest-used implantable neuroprosthetic interface. However, she explains, “researchers typically must focus on how their work will answer scientific questions, like ‘How do we modulate sensation?’” It’s more difficult to obtain enough funds to support the next, more expensive phase: clinical trials large enough to prove safety and efficacy to the FDA. Without those trials, it’s virtually impossible to attract the investment capital necessary to commercialize a new technology. And the relatively small customer base for prosthetic products makes them less financially attractive than medical innovations targeted at larger markets, such as cancer or heart disease patients.
For neuroprostheses to reach consumers, Graczyk feels two things need to happen. First, the research community must collaborate to establish standardized performance measurements for neuroprosthetics. “In the neuroscience neural engineering area, there is this problem of different people using different outcome metrics,” she says. Currently, each lab is independently creating and evaluating its own technology, which makes it difficult for funding agencies and regulators such as the FDA to gauge the relative safety and effectiveness of competing devices. “We need to come together as a community and have a discussion,” Graczyk says. Once standardized outcomes are in place, it might become easier to convince investors and manufacturers of neuroprosthetics’ profit potential.”
Second, Graczyk believes neuroprosthetic research needs to dovetail with the work occurring in the much larger community of engineers, clinicians, and manufacturers working on brain-computer interfaces (BCI). Both silos are addressing the same basic problem, translating organic nerve impulses into signals that can command and control a machine. BCI researchers are rapidly developing devices that can do everything from restoring lost vision to overcoming paralysis. And the value proposition of BCI technology is self-evident: It largely benefits patients who have almost no options for treatment, so that industry sector is a virtual blank slate. In sharp contrast, neuroprosthetics must compete in a sector that’s already dominated by conventional (and profitable) prosthetic technology.
That makes neuroprosthetics an inherently risky commercial venture, Graczyk concedes. But she adds, “If the BCI community is able to de-risk some of this and demonstrate a pathway for how to overcome the regulatory hurdles of doing these implanted devices for these kind of conditions, I think that that would actually lower the barrier to entry for limb-loss communities.”
In the meantime, research subjects like Vonderhuevel continue to demonstrate just how life-changing these bionic limbs can be. The experimental neuroprosthesis that enabled him to hug his granddaughter has been in development for nearly a decade and is now at about TRL 6. Based on that timeline, it probably won’t gain FDA approval before 2028—and market factors could further delay its arrival.
For Vonderhuevel, the value proposition goes beyond both medical efficacy and economic profitability. “If you lost your limb,” he asks, “wouldn’t you like to be able to feel it again and be able to feel what you’re touching with it?” Millions of amputees are asking the same question, and they know the answer is out there. Everyone can see it.
They just can’t quite touch it yet.