Paralyzed patients are walking again. Silent minds are finding their voice. Robotic limbs are feeling. Neurotechnology is now merging human intention with machine intelligence, transforming concepts once confined to science fiction—like the cyberspace decks from William Gibson’s Neuromancer (1984) and the neural implants from his 1981 story Johnny Mnemonic—into clinical reality. Think AI-powered brain implants that decode unspoken words at conversational speeds, to “digital bridges” reactivating paralyzed limbs through thought alone. Read on to learn more about five emerging trends—from noninvasive brain–machine interfaces to adaptive deep brain stimulation—that are steadily transforming how we restore movement, speech, and sensory feedback.
1. Brain-computer interfaces (BCIs) for motor recovery
What it is: BCIs use implanted or surface-level electrodes to record brain signals—often from the motor cortex—and translate those signals into device commands or muscle-stimulation patterns. By mapping neural intentions directly to movement, these systems bypass spinal cord damage or other neuromuscular limitations, allowing users with paralysis to regain functional motor control in real-world tasks.
Examples:
-
Paralyzed man flies virtual drone with thought-controlled finger movements
In a Stanford-led BrainGate2 clinical trial, a 69-year-old man with C4 AIS C spinal cord injury used an intracortical BCI (implanted in the motor cortex) to pilot a virtual quadcopter simply by thinking of finger movements. Real-time neural decoding translated his intentions into flight commands, allowing him to navigate through or around 18 virtual “rings” in under 3 minutes (bioRxiv preprint).
The quadcopter simulation was not an arbitrary choice; the research participant had a passion for flying.
-
Brain-spine ‘digital bridge’ enables paraplegic patient to walk again
In 2023, Researchers at CEA and EPFL developed a wireless interface that decodes signals from the motor cortex and activates spinal cord stimulation. A man, Gert-Jan Oskam (pictured at right), with complete paralysis walked, climbed stairs, and stood in social settings just by thinking about leg movement. (CEA News and Nature).This simple pleasure represents a significant change in my life
-
Double neural bypass restores arm movement and sensation
In mid-2023, Keith Thomas (New York, USA) regained the ability to move his arm by thought—and feel his sister’s touch—thanks to a combined BCI and nerve-stimulation system. Researchers routed brain signals to arm stimulators while feeding touch signals back to his brain, enabling both motor control and sensation (Feinstein Institutes for Medical Research).
-
Semi-invasive ‘NEO’ BCI reactivates hand muscles in quadriplegic man
In Shanghai, surgeons implanted a coin-sized, subdural electrode grid over the motor cortex of a 38-year-old patient with spinal cord injury as part of a clinical trial evaluating the NEO neural acquisitor and stimulator system. Within weeks, the patient was able to grasp objects and hold a cup using thought-controlled muscle stimulation. This less invasive, surface-of-the-brain approach delivers high-fidelity neural signals while reducing the risks associated with traditional intracortical methods. The patient has begun to regain motor functions—using an assistive glove to grasp objects and drink water. (medRxiv, clinicaltrials.gov).
At the 2024 Service Trade Fair, a staff member explains the brain-controlled neural rehabilitation training robot—powered by non-invasive brain–machine interface technology—to visitors. Photo by Qi Xiaoyi (source).
What’s Next
Expect expanded clinical trials (e.g., BrainGate, Onward Medical) and further refinements—such as semi-invasive “surface-of-the-brain” sensors that reduce surgical risk. Improvements in AI decoders will increase the speed and smoothness of BCI-driven movements, fueling broader accessibility, regulatory approvals, and eventual commercialization.
2. BCIs for communication and speech restoration
What it is: These BCIs tap implanted sensors in speech or motor areas, along with machine learning to decode intended words or phonemes. Whether imagining speaking, typing, or “silent” articulation, the technology maps brain signals to real-time text or synthesized voice output.
Examples:
-
Man with ALS speaks again at 97% accuracy
UC Davis researchers used a neural implant paired with AI-driven decoding to interpret attempted speech in near real-time. The patient produced sentences displayed on a screen and spoken by a digital voice—even though he had almost no audible speech (UC Davis News). Published in The New England Journal of Medicine, this approach achieved the highest-reported speech BCI accuracy to date (NEJM).
-
BCI enables real-time avatar speech
In August 2023, UCSF researchers implanted a paper‐thin, 253‐electrode array on the speech-related cortex of a paralyzed woman. This allowed her to control a digital avatar that vocalizes her intended words. The system achieved roughly 75% accuracy using a vocabulary of around 1,000 words and enabled communication at about 80 words per minute—a sizable jump compared to earlier devices that only managed 10–15 words per minute. (UCSF, UC Berkeley).
Overnight, everything was taken from me. I had a 13-month-old daughter, an 8-year-old stepson, and 26-month-old marriage.
What’s next
Refinements in language-model-assisted decoding are poised to transform “thought-to-speech” systems into more natural, conversational interfaces. Researchers expect user-friendly interfaces for daily communication—potentially integrating with smart home systems or augmented/virtual reality—making “thought-to-speech” accessible in clinical and consumer contexts.
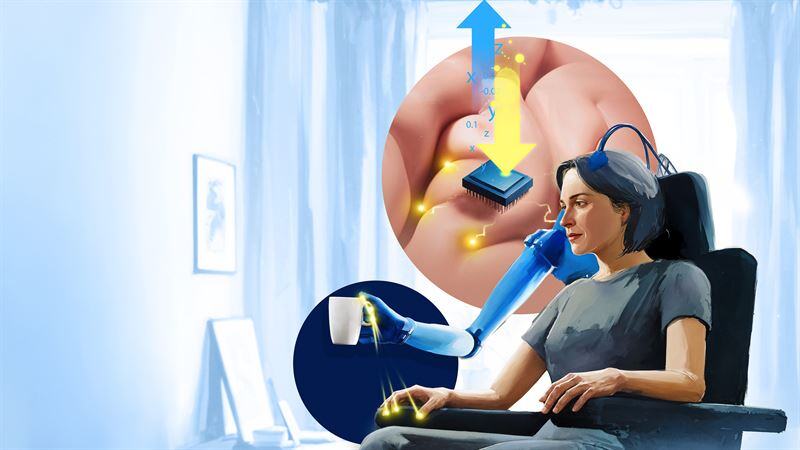
3. Neuroprosthetics with sensory feedback
What it is: Advanced prosthetic limbs interface with users’ residual nerves or muscles, providing real-time feedback about pressure, texture, temperature, or limb position (proprioception). This sensory information is delivered through electrical stimulation of the remaining nerves or skin, creating more natural and intuitive control. Feeling what a prosthetic “touches” greatly enhances usability and the sense of ownership over an artificial limb. It reduces phantom limb pain, improves fine-motor control, and restores crucial sensory cues—like feeling the weight of an object or sensing heat.
Examples:
-
Mind-controlled bionic hand with touch and temperature feedback
An osseointegrated neuromusculoskeletal prosthesis enabled Karin, a patient with a right transradial amputation—who endured over 20 years of phantom limb pain—to regain effective control of a bionic hand and perform roughly 80% of her daily activities. In this clinical study led by Max Ortiz-Catalan, titanium implants were integrated into her forearm bones, while her nerves and muscles were surgically rearranged with implanted electrodes to create a direct, bidirectional interface with the robotic Mia Hand developed by Prensilia. This system provided reliable motor control and consistent sensory feedback on her phantom limb, significantly reducing her pain while combining osseointegration, reconstructive surgery, and neural interfacing for long-term, everyday prosthesis use. (Science Robotics) -
Thermal feedback in a prosthetic hand
Researchers in Switzerland enabled a 57-year-old participant to feel the warmth of another person’s hand—a first for prosthetic limbs. They did so by integrating a portable thermal feedback system, called MiniTouch, with a commercially available robotic prosthetic hand. (Med).
What’s next
Ongoing research aims to provide multi-sensory integration (touch, temperature, texture) in lightweight, comfortable prosthetics. Surgical techniques like the agonist-antagonist myoneural interface (AMI) will see broader adoption, enabling more life-like joint movement and proprioception for both upper and lower limbs.
4. Adaptive deep brain stimulation (DBS) and closed-loop systems
What it is: DBS implants deliver electrical pulses deep in the brain to treat neurological disorders like Parkinson’s or dystonia. In an “adaptive” or closed-loop configuration, sensors and AI-based algorithms detect abnormal neural patterns—such as tremor onset—and dynamically adjust stimulation strength on the fly. This real-time personalization can minimize side effects, extend battery life, and improve symptom control.
Examples:
-
AI-guided DBS for Parkinson’s—50% reduction in ‘worst’ symptoms
UCSF scientists trialed an implanted system that adjusts stimulation based on brain signals, improving motor function and even sleep. A press release notes: “The approach, called adaptive deep brain stimulation, or aDBS, uses methods derived from AI to monitor a patient’s brain activity for changes in symptoms.” (UCSF News)
This is the future of deep brain stimulation for Parkinson’s disease
Medtronic’s Percept PC neurostimulator with BrainSense technology [Image courtesy of Medtronic]
World’s first commercial closed-loop DBS approved in Europe
Medtronic’s BrainSense adaptive DBS (aDBS) automatically personalizes stimulation in real time by monitoring neural activity, now CE-marked for Parkinson’s patients (MassDevice).
What’s next
Further FDA approvals for adaptive DBS in the U.S. and trials exploring additional indications (chronic pain, refractory depression) are on the horizon. Researchers are refining electrode design and machine-learning algorithms to tailor brain stimulation to each patient’s unique neural “fingerprints,” ushering in a personalized era of neuromodulation.
5. Wireless and minimally invasive implants
What it is: Minimally invasive implants reduce the risks and recovery time associated with traditional open-brain surgeries. Endovascular BCIs like the Stentrode reach the motor cortex via blood vessels, while fully implanted chips communicate wirelessly for power and data. Surface-of-the-brain (ECoG) arrays also minimize deep-brain penetration, lowering infection risk and surgery complexity.
Examples:
-
The Stentrode: Endovascular BCI
Synchron’s device is inserted via a blood vessel—no open-brain surgery—and records motor cortex signals to enable patients with paralysis to text, email, or control smart homes (Mount Sinai – COMMAND Trial).
Endovascular implant in a vein adjacent to the motor cortex captures movement-related signals, which are transmitted by a chest-implanted receiver to a decoder.
-
Neuralink’s fully wireless N1 implant
Elon Musk just announced that a third recipient received the its implant. Using ultra-thin electrode threads, Neuralink’s BCI sits entirely under the scalp and communicates via Bluetooth, aiming for continuous at-home use. The FDA granted the company approval in 2023 to begin its first in-human clinical trial. The N1 contains 1,024 electrodes distributed across 64 flexible threads, each thinner than a human hair (≈1/20th the width of a human hair). These threads are implanted into the brain’s motor cortex to record neural activity. The N1 contains 1,024 electrodes distributed across 64 flexible threads, each thinner than a human hair (≈1/20th the width of a human hair). These threads are implanted into the brain’s motor cortex to record neural activity. (AP).
What’s next
Further refinements in minimally invasive approaches promise to accelerate trials worldwide. As wireless power and data transmission become more robust, “take-home” BCIs and neurostimulators will transform rehabilitation, allowing continuous daily use without bulky external setups.
By Brian Buntz