The word “bionic” conjures sci-fi visions of humans enhanced to superhuman levels. It’s true that engineering advances such as better motors and batteries, together with modern computing, mean that the required mechanical and electronic systems are no longer a barrier to advanced prostheses. But the field has struggled to integrate these powerful machines with the human body.
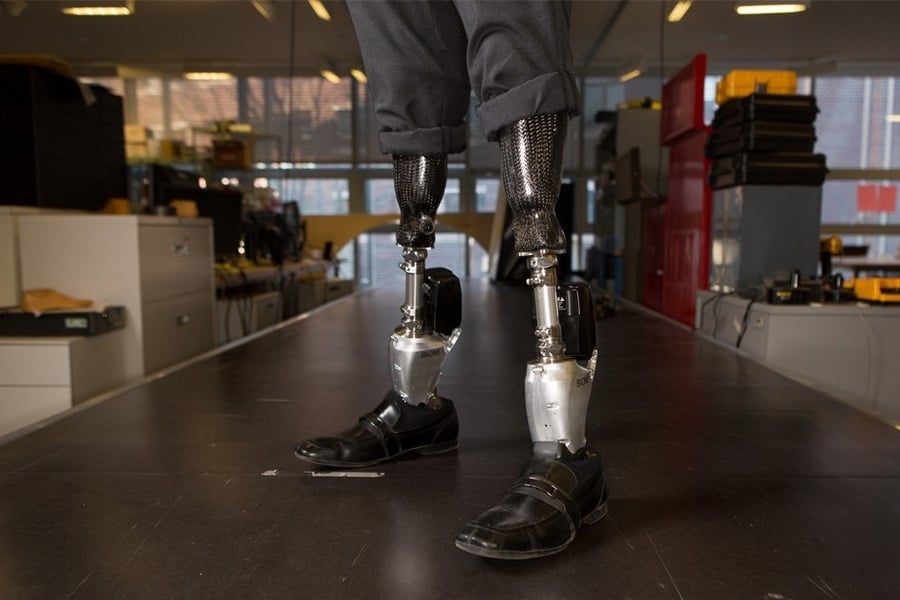
That’s starting to change. A recent trial tested one new integration technique, which involves surgically reconstructing muscle pairs that give recipients a sense of the position and movement of a bionic limb. Signals from those muscles control robotic joints, so the prosthesis is fully under control of the user’s brain. The system enabled people with below-knee amputations to walk more naturally and better navigate slopes, stairs and obstacles, researchers reported in the July Nature Medicine.
Engineers have typically viewed biology as a fixed limitation to be engineered around, says bioengineer Tyler Clites, who helped develop the technique several years ago while at MIT. “But if we look at the body as part of the system to be engineered, in parallel with the machine, the two will be able to interact better.”
That view is driving a wave of techniques that reengineer the body to better integrate with the machine. Clites, now at UCLA, calls such techniques “anatomics,” to distinguish them from traditional bionics. “The issue we were tackling wasn’t an engineering problem,” he says. “The way the body had been manipulated during the amputation wasn’t leaving it in a position to be able to control the limbs we were creating.”
In an anatomics approach, bones are exploited to provide stable anchors; nerves are rerouted to create control signals for robotic limbs or transmit sensory feedback; muscles are co-opted as biological amplifiers or grafted into place to provide more signal sources. These techniques all improve the connection and communication between a robotic limb and the human nervous system, enhancing what bionic prostheses are capable of (SN: 2/9/24).
Anatomics-based devices have been slow to make their way out of labs and into the commercial and clinical worlds. But some say the field is edging us closer to that sci-fi vision of seamlessly integrated, brain-controlled bionic limbs — especially as more advances lie around the corner.
Here’s a closer look at how researchers are aiming to marry body and machine.
Reconstructing muscles
Proprioception — the body’s awareness of itself in space — is a tricky sense to restore, but it’s important for movement, especially walking (SN: 9/9/19). Muscles send signals to our brain about where our body is, how it is moving and what forces it encounters. These signals are generated mainly by coupled muscles called agonist-antagonist pairs, where one contracts as the other stretches.
In a traditional amputation, this important feedback is discarded. But the technique reported in the July study, known as an agonist-antagonist myoneural interface, or AMI, surgically reconstructs these push-pull pairs and uses the signals they generate to control prosthetic joints. The procedure enables a recipient to “feel” their prosthetic limb.
“When the prosthesis moves, the person actually feels that movement as a natural proprioceptive sensation,” says MIT bionicist Hugh Herr, who developed the technique alongside Clites and the team’s surgeon Matthew Carty.
The recent study was part of a clinical trial that Herr and colleagues are conducting, which tested the technique in 14 people with below-the-knee amputation. Seven participants had undergone the AMI procedure, while the others had standard amputations. Recipients of the AMI-based system increased their walking speed by about 40 percent from 1.26 meters per second to 1.78 meters per second, the researchers found, a rate comparable to that of people without amputation.
Extending bones
The most common complaints from prosthetic users involve pain and discomfort. A major source of discomfort is the attachment point.
“Many of the problems with prosthetic usage are related to the socket,” says bioengineer Cindy Chestek of the University of Michigan in Ann Arbor. Squishy flesh is poorly suited to transferring loads to the part of the body built for that job — bones. The resulting strain can cause tissue damage and, invariably, discomfort, sometimes leading users to abandon their device.
A technique called osseointegration exploits the fact that certain metals bond with bone. A titanium bolt inserted into the skeleton anchors the prosthesis in place, providing greater strength, stability and comfort. “There’s a reason we have skeletons,” Chestek says.
The procedure was first carried out in 1990 but didn’t become widely accepted and clinically available until the past decade. One implant system, called OPRA, received approval from the U.S. Food and Drug Administration in 2020. The main drawback is that the titanium bolt must go through the skin, creating a permanent hole that carries infection risks. “Other than the infection risk, osseointegration is better in all ways,” Chestek says.

Rerouting nerves
Bionicists have long sought to tap into the body’s nerves to create prostheses that communicate with the brain. But early efforts were frustrating, mainly because the signals that nerves carry are very weak.
“People tried for decades to get meaningful signals from [putting] a wire inside a nerve,” Chestek says. “To this day, it’s nearly impossible outside of a controlled lab setting.”
Modern bionic prostheses communicate mostly with muscles instead. When activated by a nerve, muscles emit much larger electrical signals, which can be picked up by electrodes on the skin, which then control the prosthetic limb.
But nerves that previously operated parts of a missing limb — and could similarly efficiently operate the artificial limb — don’t usually end in muscles. They go nowhere, which creates neuromas, bulbs at nerve ends whose electrical “sparking” causes pain.
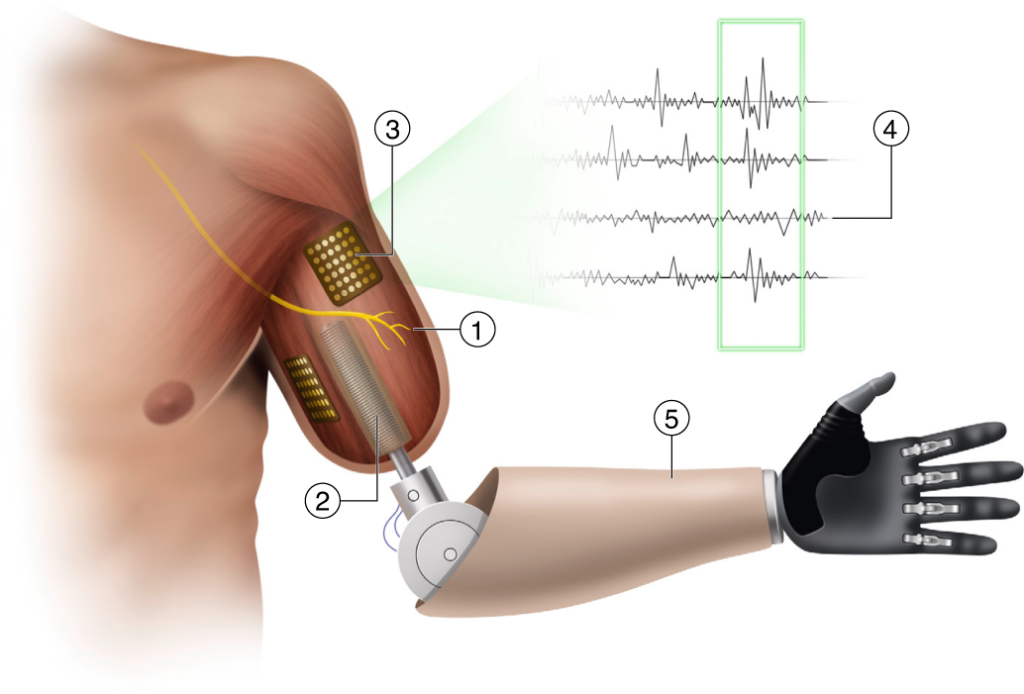
A procedure called targeted muscle reinnervation, or TMR, solves this problem. A surgeon strips muscles of their native nerves and reroutes severed nerves to this freshly cleared ground. Rerouted nerves grow into the muscles over time, which act as amplifiers, creating sources of the required control signals. “You turn a nerve recording problem into a muscle recording problem,” Chestek says. “Muscle recording is easy.” The procedure also treats neuroma pain — a purpose for which it is often carried out.
A drawback is that TMR cannibalizes existing muscles, limiting the number of signals that can be created. “You run out of real estate pretty quickly,” Chestek says. This is especially important for amputations above the knee or elbow, where there are fewer remaining muscles and more prosthetic joints to control.
A new technique, known as a regenerative peripheral nerve interface, or RPNI, surgically inserts small muscle grafts taken from elsewhere and reroutes nerves to these instead. Surgeons can then dissect these nerve bundles into their constituent fibers to capitalize on the newly grafted targets, allowing researchers to create as many signals as they need, Chestek says.
The small size of the muscle grafts makes it difficult to pick up signals from them using surface electrodes, though. “You can’t record [electrical signals] from a three-centimeter piece of muscle through the skin very easily,” Chestek says. “You have to use implanted electrodes.” This is more invasive, and implants face regulatory hurdles, but implanted electrodes produce higher quality signals. They just need to be accessed somehow, as running wires through the skin is not viable outside of laboratory studies.
Some researchers are working on wireless systems, but another solution is to combine RPNIs with osseointegration. In this setup, wires between implanted electrodes and the prosthesis simply run through the titanium bolt. A study published last year described an above-the-elbow bionic arm using this approach that enabled the recipient to control every finger of his robotic hand.
Rebuilding bodies
At his UCLA anatomics lab, Clites says, “I’ve got nine or 10 active collaborations with surgeons on different projects.” Here, he and his team use cadavers to test ideas and gather data. “We’ll mount cadaver limbs to a manipulator arm and evaluate the systems we’re developing to make sure they work as intended,” Clites says. “It’s the backbone of what we do.”
One of the projects under development is a new attachment method that avoids the permanent hole that comes with osseointegration. Instead of a titanium bolt, there’s a piece of steel in the limb and an electromagnet in the socket of the prosthetic. “That magnet holds [the socket] onto the limb,” Clites says, “and then you can control how much attractive force there is by changing the current through that electromagnet.” The socket does not have to bear loads; the magnetic force does that job, changing from moment to moment according to requirements, such as walking versus standing.

In their lab at UCLA, bioengineer Tyler Clites and Ph.D. candidate Ophelie Herve use a robot arm to manipulate a model knee in a simulation of an experiment to be conducted on cadavers.Milo Mitchell/UCLA Health
At MIT, Herr is also working on a new advance. The recent trial of AMI-based bionic legs used electrodes on the skin to shepherd signals from muscles to the prosthetic joints. But surface electrodes have drawbacks, such as movement causing signal distortions. The new technique — called magnetomicrometry — involves placing magnetic spheres inside muscles and monitoring their movement with magnetometers. “With these magnets,” Herr says, “we can measure what we care about and use it to directly control the bionic prosthesis.” A commercial product will exist in about five years, he says.
For Herr, such advances are personal. Both of his legs were amputated below the knee after a mountain climbing accident 42 years ago. He is thinking of upgrading to AMI-based bionic leg prostheses in the coming years. Once these techniques are perfected, he predicts a leap forward. “When you marry surgical techniques like AMI and RPNI with something like magnetomicrometry, we believe it’s going to be game over,” Herr says. “We believe there’s going to be the Hollywood version of brain-controlled robotic limbs.”
An added benefit of restoring proprioception, alongside other kinds of sensory feedback such as touch, is that it makes recipients feel more like a prosthetic is part of themselves (SN: 4/22/21). “The goal in the field is when we do robotic reconstruction, the person says, ‘Oh my God, you’ve given me my body back.’” Herr says. “Instead of a robotic tool, we give them a whole limb back. The field is very close to that goal.”