Hugh Herr has reached another milestone in his long quest to develop brain-controlled bionic legs.
In a study published this summer in Nature Medicine, Herr and his colleagues shared data affirming that agonist-antagonist myoneural interface (AMI) surgery—which restores broken nerve circuits in the residual limb—gives amputees unprecedented ability to control prosthetic legs with their thoughts. One study participant told CNN, “I didn’t feel like my leg had been amputated. It was the happiest moment in my life.”
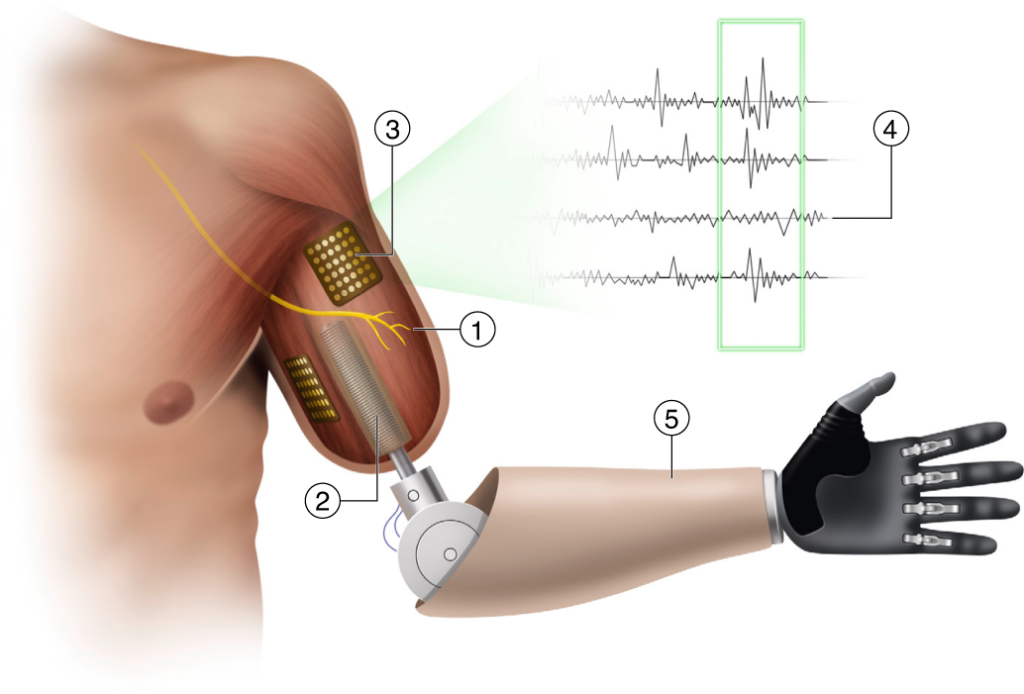
A quick refresher on AMI surgery: It’s designed to preserve natural neuromuscular relationships that are destroyed in traditional amputation surgeries. Muscles that normally contract and stretch in tandem (the agonist and antagonist) are simply dead-ended in conventional limb-removal procedures; the same goes for the nerves governing those muscles. In AMI amputations, paired muscles and nerves are rejoined, so natural patterns of sensory communication and biomechanical function remain in place. That gives amputees more intuitive control over their residual limbs and, by extension, their prostheses.
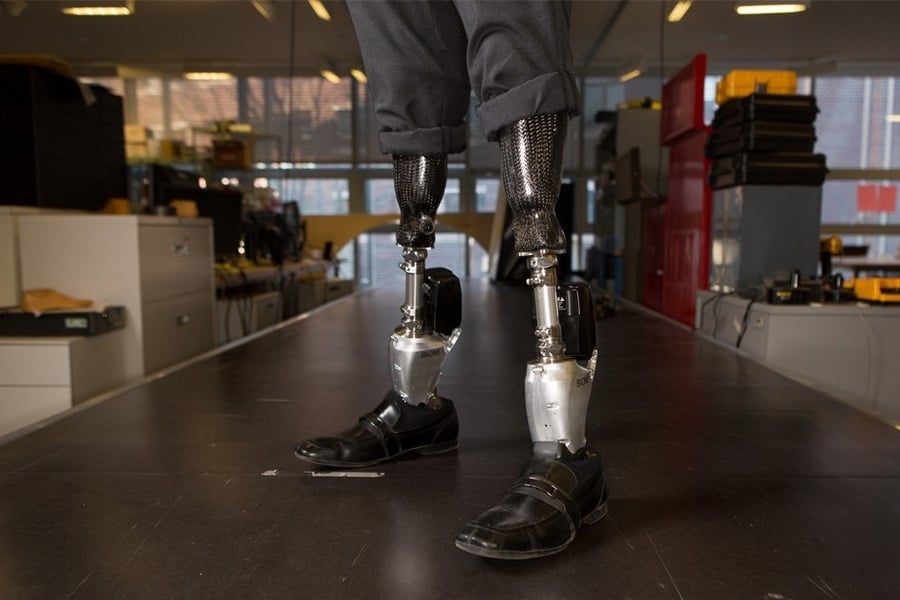
Herr has spent the last several years developing prosthetic devices that can take full advantage of an AMI limb’s robust capabilities. The prototype used in the Nature Medicine research differs significantly from conventional bionic legs, which rely mainly on positional data (speed, angle, force, direction) and pattern-recognition algorithms to produce a gait that aligns with the user’s movements. “Such intrinsic controllers estimate gait phases, speeds and environmental conditions, and then model diverse legged motions into a finite number of states,” the paper explains. “Modeling human motor intent into a finite number of states inevitably results in limiting the native versatility of legged locomotion.”
Herr’s experimental device is completely driven by the human nervous system, rather than by a microprocessor and an AI program. His prototype (a below-knee prosthesis with a powered ankle) uses surface electrodes to join the user’s residual nerve network with the device’s sensors and motors. The communication works in both directions: Wearers can receive sensory input from the device, and they can send commands to it. This system operates for all users, regardless of amputation type, but Herr’s team hypothesized that AMI amputees ought to get a better response, thanks to the neuromuscular integrity of their residual limbs.
To test that theory, study participants were divided into two groups: Half had AMI amputations, and the other half had conventional amputations. Study participants were put through a variety of paces, including level-ground walking, slope/stair transitions, obstacle crossing, and so forth. Data was gathered on walking speed, gait symmetry, overall gait biomechanics, ankle power, responsiveness to sudden transitions, and various other components of ambulation.
As hypothesized, the AMI amputees crushed it. They walked 41 percent faster than participants with conventional amputations, reaching speeds that compare favorably with nonamputee ambulators. Likewise, the AMI cohort’s gait symmetry was virtually equivalent to that of nonamputees. When walking on uneven terrain, AMI participants maintained the biomechanics of level-ground walking. During staircase exercises, they were able to modulate their prosthetic ankles to absorb shocks when descending and to maximize propulsion when ascending. They also exhibited fine motor control over the ankle when stepping over obstacles.
“Such versatile and biomimetic gait has not been attainable in contemporary bionic legs,” the paper concludes. “By considering limb reconstruction procedures as a neuroprosthetic design optimization problem, our results enable more-effective approaches to enhancing bionic limb function after amputation.”
You can read the open-access paper at nature.com.