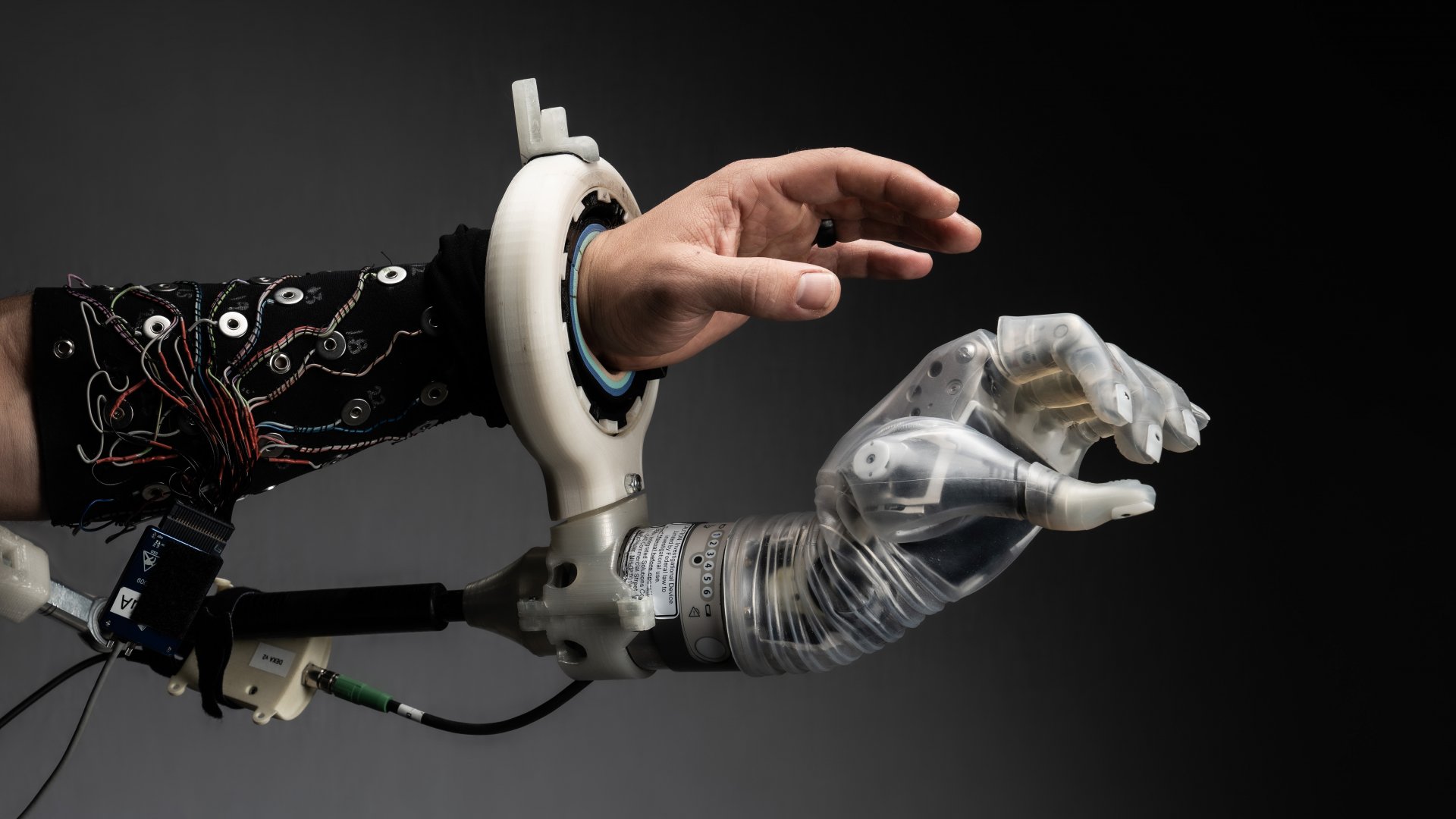
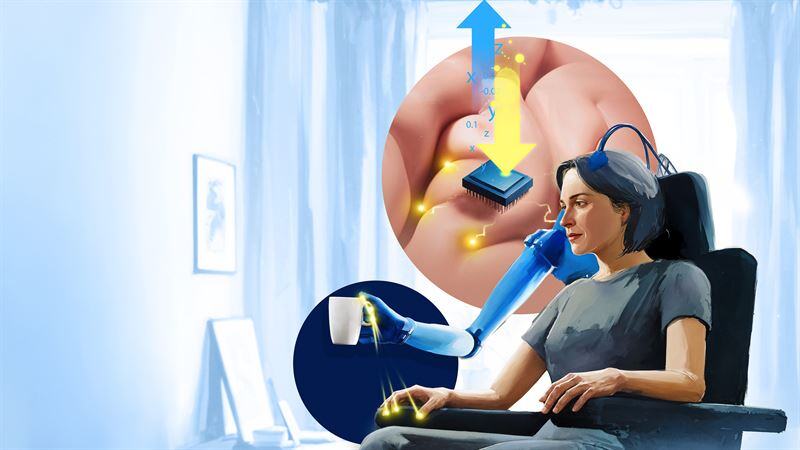
The University of Utah is preparing the LUKE Arm, a prosthetic arm and controlled by the user’s thoughts, for clinical testing in a home environment with daily use. Previous clinical trial participants primarily used the device in a laboratory setting. The university has been developing the direct nerve interface technology that enables the LUKE Arm since 2019. The multiarticulate bionic arm, developed by the company DEKA, is capable of recreating many of the movements of the human hand.
A new contract with startup Biologic Input Output Systems (BIOS) will support the university’s ongoing Investigational Device Exception Early Feasibility Study, allowing for the recruitment of the clinical trial’s next participant.
“Most importantly, this patient will be the first to take the LUKE Arm home and use it independently for their daily activities,” said Jacob George, PhD, director, Utah NeuroRobotics Lab.
George, an assistant professor in the Price College of Engineering’s Department of Electrical & Computer Engineering and the Spencer Fox Eccles School of Medicine’s Department of Physical Medicine and Rehabilitation, also serves as chief scientist for BIOS.
To enable the thought-based control of a robotic arm, enrolled patients will have Utah Slanted Electrode Arrays (USEAs) and intramuscular electromyographic recording leads (iEMGs) implanted into their residual arm nerves and muscles. After being fitted with the device in the lab and undergoing preliminary tests, the participants can then take the device home for at least one year.
In 2022, BIOS executed a licensing agreement with the university for the technology behind the neuroprosthesis and continues to move the technology toward commercialization. The original clinical trial enrolled eight participants with transtibial amputations who were supervised by engineers and clinicians on the university’s campus. With support from the Defense Advanced Research Projects Agency and the National Science Foundation, the patients helped test the bionic arm’s capabilities, including providing the user with a sense of touch.
The BIOS direct nerve interface earned a Breakthrough Device Designation from the Food and Drug Administration (FDA) and admission into its Total Product Lifecycle Advisory Program in 2024. The FDA uses these programs to fast-track promising experimental medical devices toward everyday use.
The latest iteration of the technology’s software and artificial intelligence allows for more lifelike sensation, increased dexterity, and more intuitive control, making unsupervised, everyday use feasible for the first time. The BIOS direct nerve interface can also be integrated with other technology, such as other robots, virtual reality systems, and phones/computers, so individuals can seamlessly control and feel from their devices with just their thoughts.
“We’ll now get to see how much more impactful this technology is when used daily for real-world activities,” George said. “We’re replacing the lost function of the hand, and with extended use, we hope this technology can also restore the full range of physical and emotional experiences enabled by our hands.”
The project stems from the pioneering work of emeritus faculty member Richard Normann, PhD, who invented the Utah Array, as well as computer and electrical engineering professor Florian Solzbacher, PhD, who commercialized the technology through Blackrock Neurotech. Blackrock Neurotech is a university-associated startup company that recently received a $200 million investment to expand their brain-computer interface technology.